| Host |
Anastasia Janas
|
| Editors | Matthew Baccari |
| Social Media Manager | Priya Singhal |
| Short-form Content Creator | Caitlin FitzMaurice |
| Listen | Apple Podcasts Spotify |
| Watch | YouTube |
Summary
In this episode of the Nucleate Podcast, Prof. Dr. Dominik Rüttinger takes listeners from his childhood in Munich, where a family of engineers sparked his early fascination with science, to his decision to pursue both an MD and a PhD. His interdisciplinary training, rooted in oncological surgery, gave him a unique perspective that continues to shape his career philosophy: aim high, question the status quo, and think in terms of societal impact.
Dominik reflects on his move from the operating room and academic research into the world of oncology drug development, describing the cultural and professional shifts involved in transitioning from a university hospital to the agility of biotech startups, and later to the scale and complexity of global pharma leadership. He offers a rare inside look at high-stakes decision-making in oncology R&D, highlighting the importance of humility, adaptability, and the use of unbiased frameworks to improve success rates.
The conversation also explores the evolving role of AI and machine learning in drug discovery and clinical trials, alongside the ethical and practical challenges of balancing quality of life and longevity in cancer care. Looking ahead, Dominik shares his vision for advances in prevention, early detection, and personalized medicine in the field of oncology.
The 10% Mindset: Playing the Long Game in Drug Discovery
Imagine you are the captain of a great fleet — one hundred ships gleaming in the morning sun, each representing a promising drug candidate ready to set sail. Years have gone into their construction: forging the hull (chemistry), testing the rigging (formulation), and training the crew (development teams). Charts are drawn, courses plotted, cargo loaded. Your destination: the safe harbor of regulatory approval. Between you and that harbor lies a vast, hostile ocean. Beneath the waves are reefs invisible from the deck (hidden toxicities), storms that can blow up overnight (unexpected trial results), and rival fleets racing for the same port (competitive market pressure). From centuries — well, decades — of logged voyages in FDA and EMA records, clinical trial registries, and industry analyses, you know a sobering truth: only a select few ships will survive the journey. That’s not bad luck — it’s the nature of the sea you sail. Dominik Rüttinger, Global Head of Oncology Research & Early Development at Bayer Pharmaceuticals, calls this reality the 10% mindset. It is not a pessimistic creed but a navigational chart. In his words, drug discovery is for those who do not need to be right every time, but who care about making the best possible decision at each turn of the voyage. Because the few ships that do reach harbor can transform medicine and change thousands of lives. Dominik calls this reality the 10% mindset. It is not a pessimistic creed but a navigational chart. In his words, drug discovery is for those who do not need to be right every time, but who care about making the best possible decision at each turn of the voyage. Because the few ships that do reach harbor can transform medicine and change thousands of lives.

The Gauntlet: From Target to Treatment
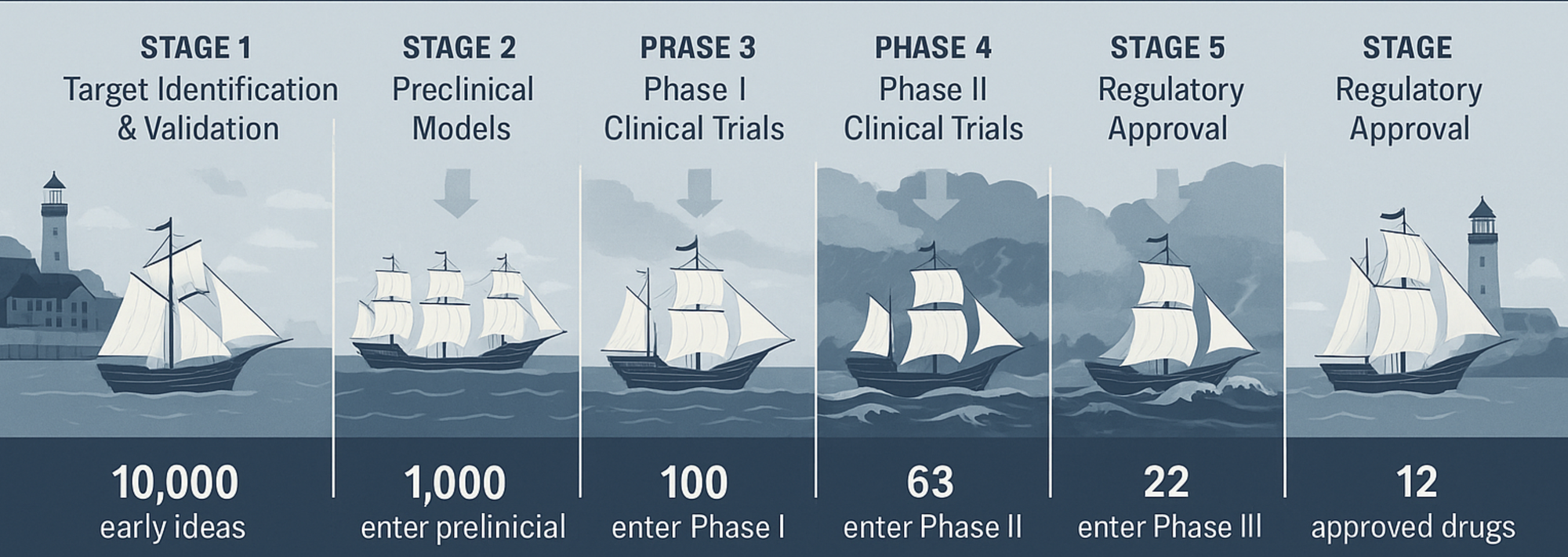
Where does the 10% come from? Why not any other, better, number? The figure isn’t a guess — it’s the product of decades of industry-wide attrition data. To stay in the ship analogy, every voyage begins the same way: ships in harbor, sails furled, crews ready. But only some will survive the gauntlet: a series of treacherous straits, reefs, and storm belts that thin the fleet at every turn.
Stage 1 – Target Identification & Validation
Before a ship even leaves the shipyard, you decide where it’s going. Thousands of potential harbors (biological targets) are charted each year, but most are mirages: poor biology, undruggable structure, or lack of relevance to disease. In the real world, this is where thousands of early concepts — hits from screening campaigns, literature-derived hypotheses, or AI-predicted targets — are filtered. Without strong validation (human genetics, pathophysiology, translational evidence), the ship risks being doomed from the start. The 10% mindset demands ruthless cartography here: you kill bad routes early, no matter how beautiful the sails look on the blueprint.
Stage 2 – Preclinical Models
Now a smaller convoy leaves the dock — dozens rather than thousands. Each must prove its hull (the drug’s chemistry) and rigging (the formulation) can survive in controlled waters: in vitro systems, animal models, pharmacokinetics, toxicity testing. The attrition is brutal: ~89–90% sink here, never reaching human waters. A common killer is poor “drug-likeness”: instability, insolubility, off-target toxicity. And sometimes the problem lies not in the ship but in the testing sea itself: the chosen model may not reflect the true conditions of human disease, leading promising vessels to founder in artificial waters. Ships that make it through earn the right to file an Investigational New Drug (IND) application: the maritime equivalent of getting your papers checked before leaving territorial waters.
Stage 3 – Phase I Clinical Trials
This is the first open-water test in human seas: small, close to shore. Crews are tiny: a few dozen healthy volunteers or, in oncology, patients with advanced disease. The question is not will the ship win the race, but will it float and handle predictably. Success rates here are ~60–70%; around one in three still flounder due to unexpected toxicity, intolerable pharmacology, or poor pharmacokinetics. Even with perfect preclinical navigation, some reefs are invisible until you’re at sea.
Stage 4 – Phase II Clinical Trials (The Storm Belt)
The most feared part of the journey. The fleet sails into heavier seas — hundreds of patients, multi-center trials — where the true performance is tested against disease.
Historically, this is the lowest survival point: ~30–40% make it through. Around 60–70% sink, most from the same cause: the drug simply doesn’t work well enough in humans. Sometimes the wind dies (weak efficacy signal), sometimes a storm tears the sails (safety issues), sometimes the current is wrong (dose, patient population). This is the “valley of death” for drug programs. It’s also the point where AI-guided enrichment and adaptive trial designs can lift survival odds, making this stretch a prime target for innovation.
Stage 5 – Phase III Clinical Trials
Ships that clear the storm belt face the longest leg: multi-year, global trials with hundreds to thousands of patients. The question now is can this ship carry valuable cargo across an ocean and deliver it reliably.
Despite earlier filtering, 30–50% still sink here — often from efficacy signals that don’t replicate at scale, rare safety events emerging in large populations, or a competitor reaching the harbor first and raising the entry bar.
Stage 6 – Regulatory Approval
Survivors of Phase III reach the harbor walls and must convince the port authority (FDA, EMA) they are seaworthy. At this point, most do: ~85–90% approval rates. Failures here tend to be from manufacturing issues, inadequate evidence, or strategic withdrawals.
The Economics of the Voyage: each leg costs more than the last. By the time a fleet finishes, the average cost to bring a single ship (drug) from target ID to port now runs $1–3 billion and takes 8–15 years, even with modern accelerators. Each wrecked ship’s cost is carried by the few that make it — one reason prices are high and portfolios are broad.
Why Ninety Ships Never Arrive: Bias and Blind Spots
Some ships never make it because of the sea itself — biology’s unknown unknowns. But others sink because of the captain’s own blind spots. Dominik warns that the difference between the 90% that fail and the 10% that succeed often comes down to recognizing and correcting for these blind spots early.
The most treacherous of these is confirmatory bias. You’ve invested years in a vessel, so you want to believe she’s sound. You ignore the subtle groan of her timbers (a borderline efficacy readout), the tiny leak just above the waterline (a toxicity signal), convincing yourself these are nothing to worry about. Then, halfway through a gale, the hull gives way, a perfect storm for setting them up for an expensive Phase II/III crash.
Equally dangerous is portfolio bias. Perhaps you’ve grown enamored with one particular route — a mechanism of action — and send too many ships that way. If a storm closes that passage (say, a competing therapy launches or the class fails on safety grounds), you lose not one ship but a swath of your fleet.
Dominik’s answer is cultural: build a fleet where data, not ego, determines the course. Celebrate the decision to recall a ship before disaster; see it as prudent seamanship, not defeat.
Inspection Harbors: STOP/GO Gates
Before any ship dares the next leg, it docks in a quiet harbor for a full inspection. These STOP/GO gates are where the hard calls are made.
You and your officers examine the charts and logs:
- Specificity of the target: Is the compass true? Is the therapy hitting only the target intended, or is it drifting into dangerous off-target waters?
- Translational models: Were the sea trials realistic? Did the preclinical models truly mimic the swells and currents of human disease?
- Safety & tolerability: Can the crew endure the next leg? Tolerability isn’t just a nicety — a ship that breaks its sailors (patients) will never finish.
- Competitive landscape: Will the cargo be valuable when we arrive? Even a flawless crossing is worthless if the port is already flooded with similar goods.
Here, “stop” is the default order. “Go” is earned with evidence.
The Master Cartographer: AI as an Accelerator
In Dominik’s world, AI is the master cartographer — not the captain, but the one who can map out millions of possible routes before you untie from the dock.
The cartographer can:
- Sketch detailed sea charts of chemical space, screening compounds virtually before the first lab experiment
- Mark alternate ports — adjacent mechanisms — in case your primary destination becomes unreachable
- Forecast storms weeks out, predicting toxicity or poor pharmacokinetics from early data
- Advise on convoy formation, loading the most promising patient subgroups for the trials ahead
- Dust off old maps for proven safe ships and plot them new courses to untapped ports (repurposing)
But just as a cartographer is only as good as his surveys, AI is only as good as the data it’s fed, and only once you’ve decided which vessel to build. Biased or incomplete datasets can produce exquisitely detailed schematics that still leave you with a fragile hull. Equally misguided, is to design the blueprint before deciding what voyage it must endure, or to assume the schematics alone will carry you to approval harbor without human judgment at the helm i.e. poor underdeveloped models.

Choosing the Right Ship: Target Identification
Every voyage begins with selecting the vessel itself. In drug development, that’s target identification — one of the highest-risk decisions.
Pick the wrong ship, and no matter how skilled the crew or favorable the weather, the vessel will never survive the open sea. Worse, you may only discover after launch that the hull is fatally flawed — the biology irrelevant, or the mechanism fundamentally undruggable.
Dominik’s “triangle” of safety here is a high-quality dataset, a well-formed hypothesis, and AI’s exploratory power. Together, they can:
- Stress-test whether the ship is seaworthy before launch
- Rank vessels by resilience — how druggable the target truly is
Reverse-engineer past successful voyages to learn which ships endured, and why they delivered valuable cargo.
Case log
Rivaroxaban (Xarelto): the convoy that sequenced its ports
A direct Factor Xa inhibitor launched into rough anticoagulant waters after another ship in the class (ximelagatran/Exanta) wrecked on the reef of hepatotoxicity. The program managed risk via portfolio design and partnership (Bayer with J&J) and by sequencing harbors: first short-haul orthopedic thromboprophylaxis (RECORD trials), then the trans-oceanic ROCKET-AF voyage in atrial fibrillation, then additional indications.
- STOP/GO logic: Phase II dose-finding showed the hull could take the strain (no ximelagatran-like liver signal), earning a “GO” for multiple Phase III passages
- Competitive current: The bar was “non-inferiority vs warfarin” with operational advantages (fixed dosing, no INR monitoring). The cargo was clearly valuable on arrival
- Mindset lesson: Diversify routes, spread risk with partners, and expand indications only once the first harbor is secured
Asundexian: a modern frigate that met a stronger current
A Factor XI inhibitor designed to reduce thrombosis with less bleeding: a mechanistic bet on decoupling efficacy from hemorrhagic risk. Early legs (Phase I/II) were smooth, prompting ambitious, parallel mega-voyages: OCEANIC-AF (~18k patients) and OCEANIC-STROKE (~8k). An interim look in AF signaled futility vs Eliquis (apixaban), and the AF voyage was aborted.
- STOP/GO logic: A textbook early recall in Phase III when headwinds became overwhelming; better to beach the ship than burn further capital
- Competitive current: Standards of care had advanced; the harbor’s value threshold rose mid-voyage
- Bias control: Avoid “sunk-cost sail-on.” The decision showed adherence to the 10% mindset’s prime directive: protect the fleet
Vilaprisan: a sloop that found a hidden reef in long-haul testing
A selective progesterone receptor modulator for uterine fibroids progressed well in early seas but parallel long-duration toxicology (the safety soundings you run while still underway) flagged preneoplastic changes in rodents. The voyage stopped before the deep ocean.
- STOP/GO logic: Negative preclinical carcinogenicity is a hard “STOP,” regardless of early clinical promise
- Mindset lesson: The ocean hides reefs you cannot see from the deck; build procedures (parallel tox) that find them early. Celebrate the recall
Amikacin Inhale: when a clever rig can’t beat headwinds
An inhaled amikacin plus nebulizer system for ventilator-associated pneumonia promised higher lung exposure with less systemic toxicity. Phase III (INHALE program) missed primary and secondary endpoints against standard intravenous therapy.
- Translational gap: Elegant in vitro deposition and modeling did not fully translate into clinical delta at the bedside
- Design inflection: Against potent background antibiotics, the add-on signal needed to be large; it wasn’t
- Mindset lesson: If the port already trades in similar cargo at low cost (effective SOC), your arriving goods must be indisputably better
Sorafenib (Nexavar): the mid-ocean course correction
Initially pointed at colon cancer’s crowded harbor, the ship changed destination mid-voyage toward renal cell carcinoma and hepatocellular carcinoma when early signals suggested stronger winds there. It ultimately docked as the first effective targeted therapy in advanced liver cancer.
- Target/indication agility: Same hull, different port; biology-matched routing made the difference
- Mindset lesson: Do not cling to your first map. Replot when currents (biology, signals, competitor moves) suggest a better harbor

The 10% Mindset at the Helm
Dominik’s philosophy is simple: as captain, you must accept that most ships will not make the crossing. Your job is to run a disciplined fleet:
- Inspect relentlessly at each harbor = They’ve been stress-tested in diverse models before entering the clinic
- Prune early to save resources for ships that can finish = Their teams are disciplined enough to walk away when the data turns negative.
- Diversify routes so one storm doesn’t take your whole fleet = Reduce bias in your process
The 10% mindset is not about mourning the 90 ships that don’t make it—it’s about valuing and protecting the ones that do. For Dominik, this work is for captains who find satisfaction not in being “right” about every ship, but in building fleets, processes, and strategies that make those rare arrivals transformative. The goal is not to be right about every voyage, it’s to ensure that the ships that do arrive carry cargo that changes the world.
Links
Dominik's Linkedin: https://www.linkedin.com/in/dominik-ruettinger-57395910/
Bayer Pharmaceuticals: https://www.linkedin.com/company/bayer/










