| Host |
Sam Kessel
|
| Editors | Priya Singhal |
| Long-form writer | Alex Mandia |
| Social Media Managers | Priya Singhal Teresa Tenggono Jacquelyn Hilber Marina Khovedashian |
| Short-form Content Creator | Xiong Yufan |
| Listen | Apple Podcasts Spotify |
| Watch | YouTube |
Summary
In this episode of the Nucleate podcast, Sam Kessel interviews Joe Horsman, an investor at Madrona, about his unique career path from academic researcher to biotech operator and ultimately to venture capital. Joe shares his experiences growing up in Seattle, pursuing a PhD in biochemistry, and transitioning into industry roles at NanoString, Stratos Genomics, and Roche. He discusses the evolution of the Seattle biotech ecosystem, highlighting its strengths in research, the influence of major tech companies, and the challenges and opportunities that come with building innovative companies in an emerging hub.
Joe also delves into the impact of cutting-edge DNA sequencing technologies and the increasing integration of AI and data science in biotech. He explains Madrona’s investment thesis at the intersection of life and computer sciences, emphasizing the importance of proprietary data, founder-led companies, and a focus on solving meaningful problems. Throughout the conversation, Joe offers practical advice for entrepreneurs, encouraging them to seek diverse perspectives, rigorously test their ideas, and remain optimistic and persistent, especially in challenging funding environments.
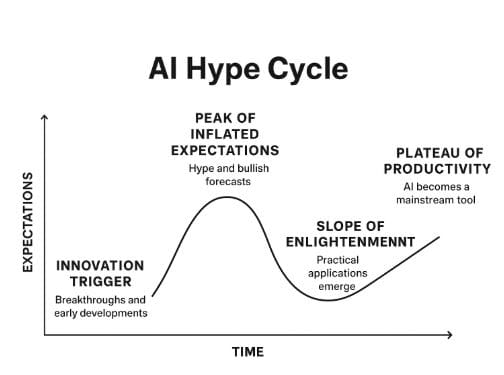
Navigating the AI Hype Cycle in Healthcare and Biotech
In a recent discussion, Joe Horsman highlighted that while AI's immediate impact might be overestimated, its long-term potential over the next decade is likely underestimated. This underscores the importance of discerning genuine advancements from mere hype, especially in the investment landscape, where AI's promise to transform industries like healthcare and biotech is significant, yet accompanied by new risks. Horsman advises investors to look beyond superficial AI claims and evaluate a company's true capabilities, data foundations, and scalability. For operators, integrating AI into R&D or clinical workflows is complex, with success depending on integration, regulatory acceptance, and cultural adoption, not just model quality. The impact of AI will be uneven, leading to a few successes amidst many ventures that fail to deliver on their ambitious promises.
Challenges in AI Adoption
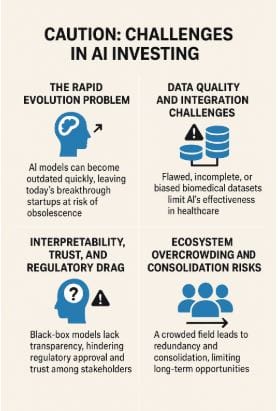
Adopting AI in healthcare and biotech faces several challenges. The rapid evolution of technology means that today's cutting-edge models can quickly become obsolete, as seen with DeepMind’s AlphaFold, which commoditized protein structure prediction. This poses a risk for startups built on proprietary models that can be easily replicated or surpassed by open-source alternatives. Data quality and integration are also critical; as Horsman emphasizes, "you need to find credible people who are working toward credible problems with credible datasets." The failure of IBM Watson Health, due to incomplete and inconsistent data, serves as a cautionary tale. Biomedical datasets are often fragmented and biased, making effective integration a significant hurdle for startups.
Overcoming Hurdles and Emerging Opportunities
The "black box" nature of many AI models hinders adoption in regulated industries, as regulators and clinicians demand interpretability, especially when patient safety is at stake. The slow pace of regulatory frameworks compared to rapid AI development creates uncertainty. The AI-in-biotech landscape is also becoming overcrowded, with many startups vying for similar solutions, leading to inevitable consolidation. However, there are signs of relief. Horsman suggests that the quickest wins will come from enhancing, not replacing, existing lab processes. AI is already proving valuable in repurposing libraries, protein modeling, and omics-driven analysis, with companies like Roche investing in closed-loop systems that integrate wet-lab data with AI models to accelerate discovery.
The Future of AI in Health
Beyond the lab, AI is transforming antibody development, drug discovery workflows, and clinical trials by optimizing patient matching, monitoring safety signals, and creating digital twin control arms. AI is also reshaping personalized health through wearable data analysis and sensor-based models that predict health outcomes and offer personalized guidance. Despite these advancements, questions of validation, equity, and data privacy remain. Ultimately, separating real opportunity from hype requires focusing on fundamentals and long-term viability, as emphasized by Horsman. In this rapidly evolving field, careful evaluation is crucial to distinguish durable opportunities from those that will fade.